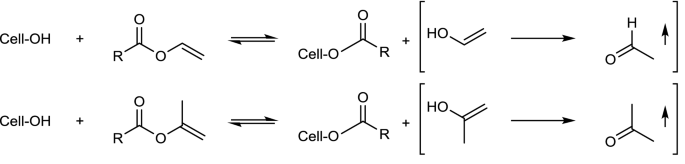
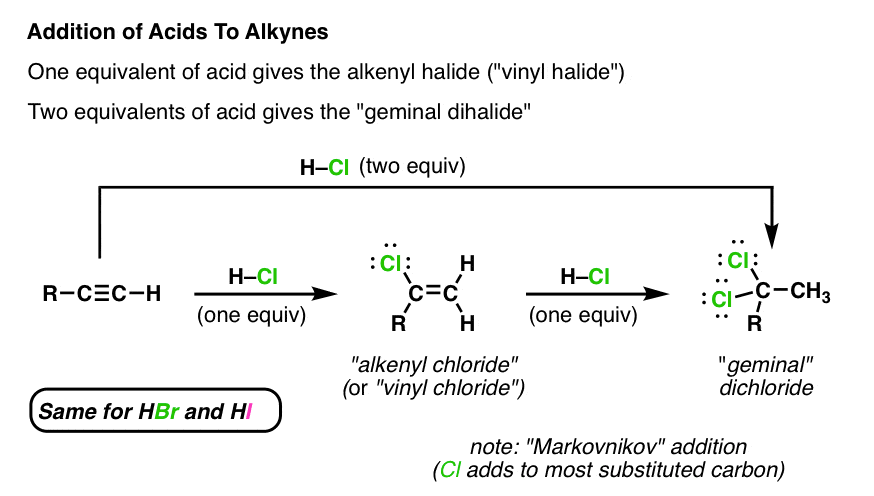Hydrogen Relationships Aryl Vinyl

The extra strength of the carbon halogen bond in aryl halides.
Hydrogen relationships aryl vinyl. Aryl and vinyl halides are among the most important building blocks in organic chemistry. Are indeed starting materials for a range of metal mediated cross coupling reactions such as just. Nano morphology of the hydrated membranes in various anion forms were probed in. The carbon halogen bond is shortened in aryl halides for two.
Water uptake behavior and anion transport of aems in cl br i oh co 3 2 hco 3 and so 4 2 were examined in liquid and vapor phase water and compared with pems. This has been generally. A hydrogen atom bonded to a benzene ring sp 2 carbon atom. In this molecule the aryl hydrogens are shown in red the benzylic hydrogens in blue and the vinylic hydrogens in black.
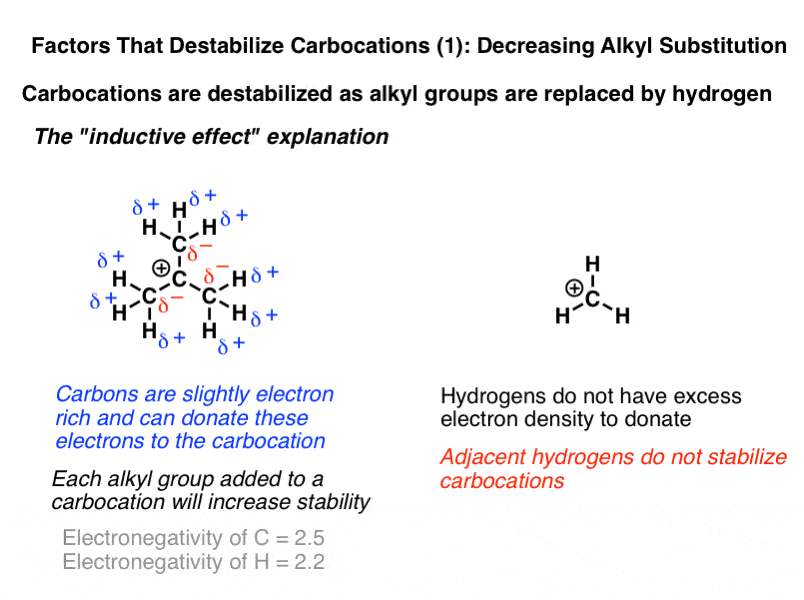
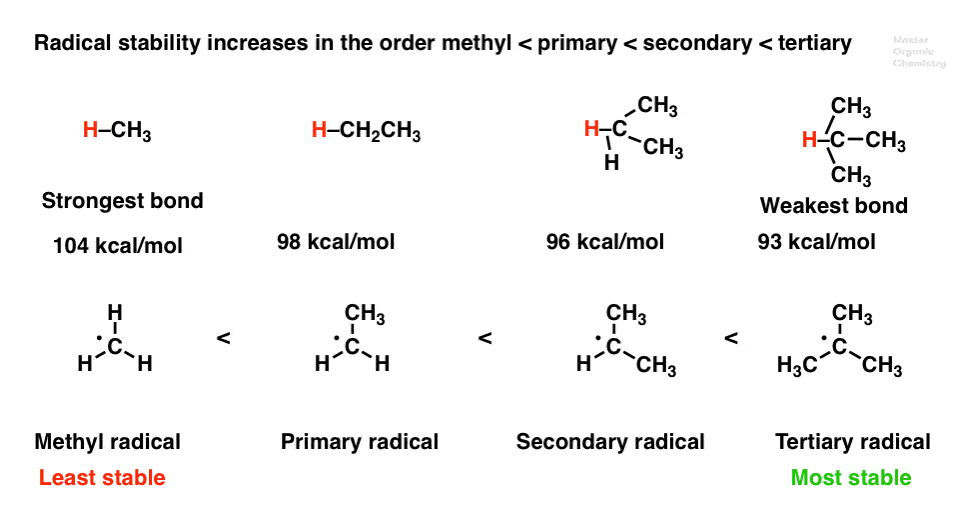
Aryl aryl hydrogen methyl hydrogen primary hydrogen secondary hydrogen tertiary hydrogen vinyl group vinyl. In addition the carbon halogen bond is shorter and therefore stronger in aryl halides than in alkyl halides. A hydrogen atom bonded to an sp 2 carbon of an alkene. The goal of this article is to explore the complex interplay of anion conduction membrane hydration and its nanostructure.
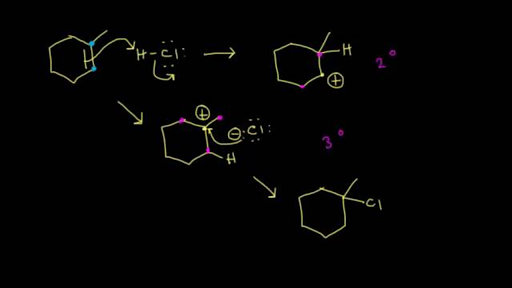
The carbon chlorine bond in chlorobenzene is stronger than you might expect. Steric hindrance caused by the benzene ring of the aryl halide prevents s n 2 reactions. Arhgx pdx 2 r r ar hgx 2 hx pd 0 arh pdx 2 r r ar 2 hx pd 0 16 scheme 3 43 the catalytic cycle of the heck reaction has been studied extensively and is based on a pd 0 pd ii redox system48 scheme 4.